Monik Sheth, Co-Founder & CEO of Ultralight Labs
Oct 8, 2024
Many medical device startups struggle with or outright neglect effective risk management. This needlessly leads to costly delays, recalls, and even compliance failures. Without a solid risk management process, even the most innovative devices can fail to make it to market or, worse, harm patients.
For medical device startups, ensuring product safety and efficacy is not just a regulatory requirement—it’s a foundational aspect of building trust with healthcare providers and patients. Effective risk management helps identify, evaluate, and control potential risks throughout the product lifecycle, reducing the likelihood of failures or adverse events.
In this guide, we’ll cover the basics of risk management, referencing the latest FDA guidance and key processes such as ISO 14971 and Failure Mode and Effects Analyses (FMEAs).
Key Risk Processes
To manage risk effectively, medical device companies often use two fundamental tools: ISO 14971 Hazard Analysis and Failure Mode and Effects Analysis (FMEA). These methods help ensure that any potential risks to patient safety or device performance are identified, evaluated, and controlled throughout the device lifecycle.
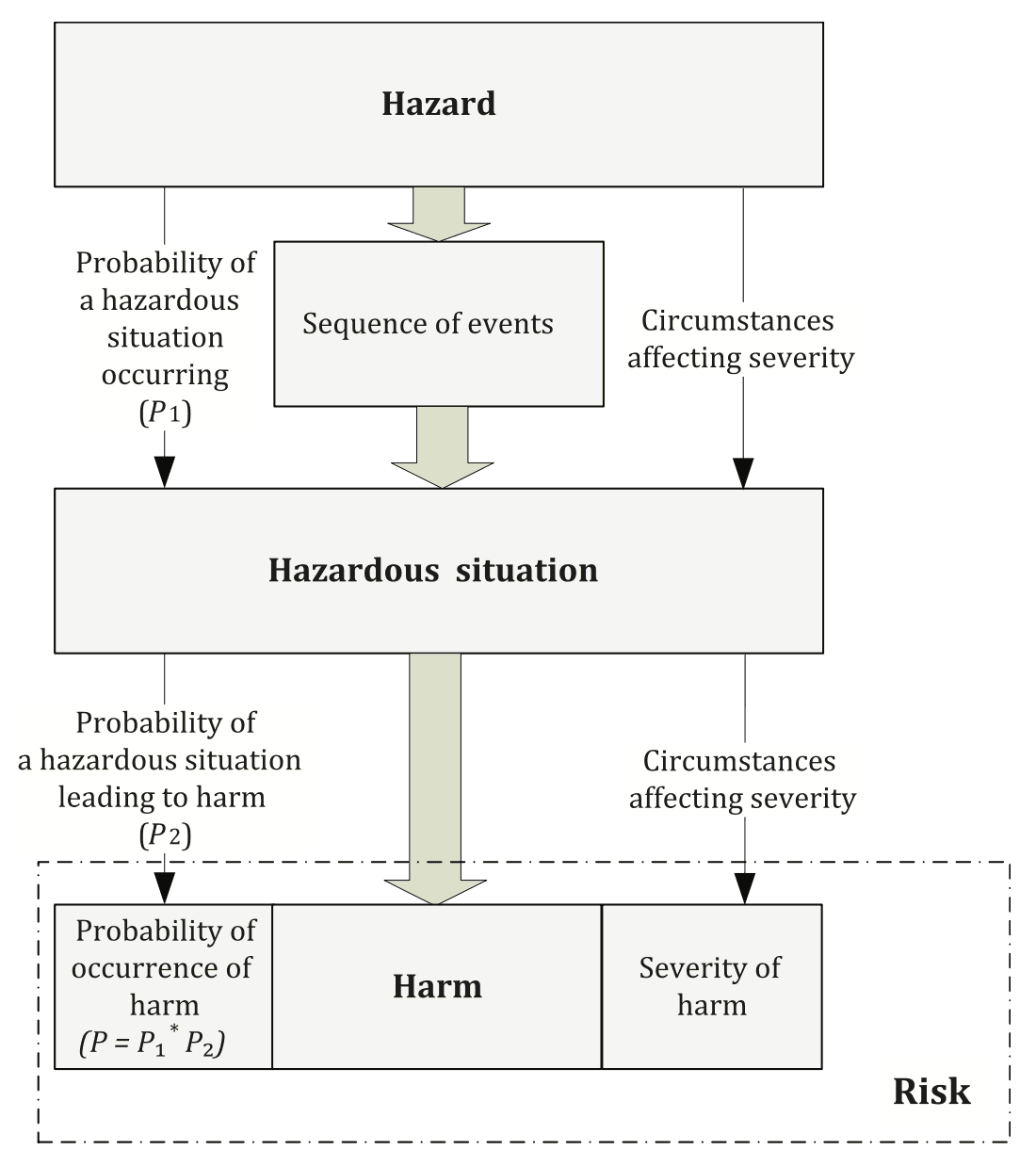
Annex C in ISO 14971:2019
ISO 14971 is the internationally recognized standard that provides a structured approach to managing risks associated with medical devices. Its framework requires companies to systematically identify potential hazards, evaluate risks, and implement controls to mitigate those risks.
The hazard analysis process under ISO 14971 involves the following steps:
Identifying Hazards: List all potential hazards that could pose risks to device safety or performance, such as user errors, environmental factors, and hardware/software malfunctions.
Assessing Risk: Estimate the likelihood and severity of harm for each hazard. This helps prioritize risks and determines which ones require immediate attention.
Implementing Risk Controls: Design and implement measures to reduce risks to an acceptable level. Controls may involve design changes, procedural safeguards, or user training.
Evaluating Residual Risk: After applying controls, assess any remaining risks to ensure they are within acceptable limits. This process should continue throughout the device’s lifecycle, including post-market surveillance.
ISO 14971 ensures that startups systematically address all potential risks from the earliest stages of development, providing a clear pathway to regulatory compliance and safe, reliable products.
Failure Mode & Effects Analysis (FMEA)
FMEA complements ISO 14971 by taking a deeper, more detailed look at potential failure modes within a device or system. This tool identifies specific ways components or systems could fail and assesses their effects on device performance and safety. Contrary to a typical ISO 14971 Hazard Analysis, FMEA is a bottom-up approach, meaning that it starts at a low level of the product or process, working its way up to the effects to the system of subsystems.
The key steps in an FMEA include:
Identifying Failure Modes: List all possible failure modes for each component or system within the device.
Analyzing Effects: Evaluate the potential impact of each failure mode on device function, safety, or usability.
Assessing Likelihood and Detectability: Estimate how likely each failure mode is to occur and how easily it could be detected before causing harm.
Prioritizing Risks: Use the Risk Priority Number (RPN) to rank failure modes based on their severity, likelihood, and detectability.
Implementing Mitigations or Controls: For high-priority risks, develop strategies to mitigate or prevent failures, such as design changes or enhanced testing protocols.
Practical Applications of Risk Management
During Product Development: Startups can integrate risk management into the design process to address safety and performance risks early, minimizing costly changes later and speeding up regulatory approval.
In Manufacturing: Ongoing risk management is essential in manufacturing, where startups can identify and mitigate risks like material defects, assembly errors, or contamination.
Post-Market Surveillance: Risk management doesn’t stop after launch. Startups must continuously monitor devices in the market, using real-world data to update their risk files and implement further controls as needed.
Latest FDA Guidance on Risk Management
The FDA strongly encourages medical device companies to adopt ISO 14971 as the foundation for their risk management practices. Recent guidance emphasizes the importance of thorough risk documentation, traceability of risk controls, and ongoing risk assessment throughout the device’s lifecycle. Startups that implement strong risk management processes early on can avoid costly mistakes and ensure smoother regulatory pathways.
How Software Tools Can Help
Managing risk manually or using spreadsheets can be cumbersome, especially for startups with limited resources. Software tools can significantly streamline risk management by automating many of the processes involved, ensuring accuracy, and making it easier to maintain a comprehensive, up-to-date risk management file.
Here’s how software tools can help:
Traceability: Software platforms can ensure that all risk controls are traceable throughout the product lifecycle, linking risks to specific design requirements, test cases, and post-market data.
Automated Risk Analysis: Automated risk assessment tools help startups quickly analyze failure modes and hazards, prioritize risks, and implement controls without manual calculation errors.
Real-Time Monitoring: Risk management software can continuously track risks in real-time, alerting companies to any issues that require immediate attention, such as adverse events or user feedback.
Compliance: Many software platforms are designed to meet the requirements of ISO 14971 and other regulatory standards, ensuring that startups stay compliant with FDA regulations.
When evaluating solutions for risk management, medical device teams should ensure the following:
Ability to handle multiple types of risk analyses, including ISO 14971 Hazard Analysis and FMEAs.
Configurability to add custom fields to map to your preferred processes.
Data flexibility such that it’s easy to export all of your documents and data, anytime.
Automatic document generation.
Easy data visualization and organization capabilities to manage large and complicated data sets.
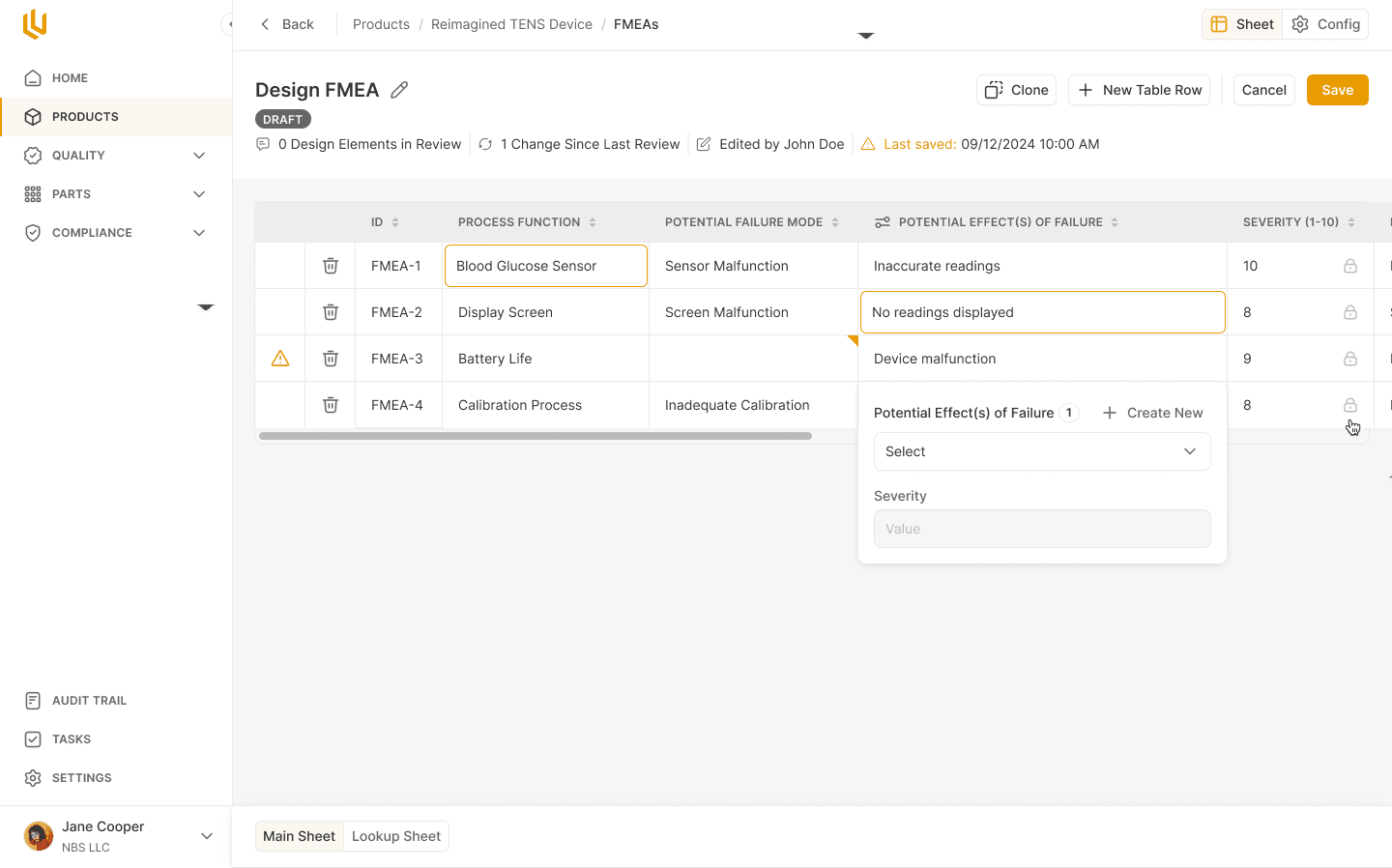
Select Screenshots from Ultralight's Risk Management Hub
At Ultralight Labs, we provide a modern eQMS platform designed to help medical device startups manage risks efficiently and effectively. Our platform integrates risk management tools that align with ISO 14971 and streamline processes like hazard analysis and FMEA, ensuring you can focus on innovation while maintaining compliance. Learn more about how our platform can support your risk management efforts at www.ultralightlabs.com.
Reach Out!
Risk management is essential for developing safe and compliant medical devices. By using structured processes like ISO 14971 Hazard Analysis and FMEA, medical device startups can systematically address potential risks, ensuring a safer product and a smoother path to market.
At Ultralight Labs, we help startups streamline their risk management processes with modern tools that integrate seamlessly into your quality systems. Whether you're just starting or optimizing an existing process, we’re here to help.
Want to dive deeper? Reach out and we’ll walk you through how we’ve helped dozens of innovative teams implement more streamlined processes for risk management. Book a demo here.


