Monik Sheth, Co-Founder & CEO of Ultralight Labs
Nov 20, 2023
One advantage of AI/ML-enabled medical devices is that they can quickly improve based on post-commercialization learning. Thus the FDA has adopted a Predetermined Change Control Plan (PCCP) regulatory framework for AI-ML-enabled medical devices, which enables certain pre-approved modifications without the need for further FDA submissions. This post summarizes this major opportunity for AI/ML product teams.
The FDA’s Framework for AI/ML Changes
The FDA’s mission is to protect the public health, and what’s implied in that mission is to not unnecessarily stifle innovation that could lead to improved health outcomes. In 2023, one of the most explosive categories of innovation is artificial intelligence & machine learning (AI/ML), which has touched many industries including healthcare.
While regulatory frameworks for AI/ML are broadly being established and are expected to evolve across industries and geographies, the FDA has already authorized more than 500 AI/ML-enabled medical devices, and is expected to authorize nearly 200 in 2023 alone.
In order to embrace the fast iteration of AI/ML, the FDA has adopted a Predetermined Change Control Plan (PCCP) framework.
A PCCP is essentially a document, to be included with FDA marketing submissions, which describes intended AI/ML software modifications and how they’ll be assessed by the sponsor. By submitting a PCCP, a device sponsor may obtain pre-approval for these intended modifications without needing follow-on submissions.
Great! But how might this work in practice?
PCCPs in Practice
In July 2023, Apple was granted a 510(k) for the algorithm that powers the Apple Watch’s ability to let users get an electrocardiogram directly from their wrists. In this submission, Apple included a PCCP which includes a list of potential software modifications, associated requirements, and test methods. You see the summary PCCP table below and view the full 510(k) summary here.
PCCP for the Apple Watch’s Irregular Rhythm Notification Feature, July 2023
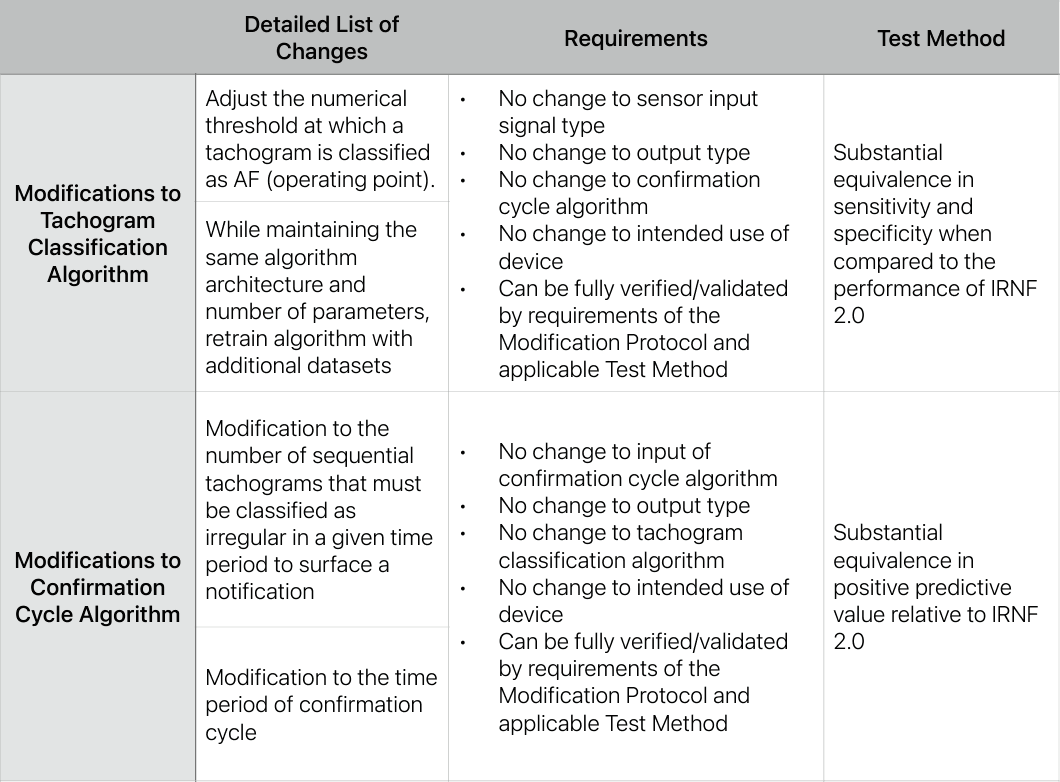
In order to help AI/ML device sponsors with implementation, the FDA has also identified 5 guiding principles for PCCPs in October 2023, summarized below.
Focused and Bounded: PCCPs target specific, limited changes within the device's intended scope, with plans for safe modification and quality assurance.
Risk-based: PCCPs are guided by risk management throughout the device's lifecycle, ensuring long-term safety and appropriateness of changes.
Evidence-based: Safety and effectiveness of PCCP changes are backed by continuous, lifecycle-wide evidence, balancing benefits and risks.
Transparent: PCCPs require clear communication about changes, testing procedures, and performance monitoring to stakeholders.
Total Product Lifecycle Perspective: PCCPs integrate stakeholder and risk management views throughout the device's lifecycle, maintaining safety and regulatory compliance.
How Ultralight Can Help
At Ultralight, we’ve built the most intuitive requirements and quality management system tailored towards modern product teams. Working alongside leading AI/ML-enabled medical device companies, we’ve designed our software platform to allow engineering teams to take advantage of all the benefits of agile development while staying compliant with regulatory requirements and designing a safe and effective device.
Specifically, there are three main ways our customers can leverage Ultralight platform to help them manage their PCCPs:
Configurable Change Control Workflows: A common trade-off in software for change control is between how flexible versus rigid the tool is. At Ultralight, we’ve aimed to strike the right balance, in order to ensure an easy starting point while still allowing for appropriate configurability to account for the unique needs of any particular organization. Ultralight has pre-built change order templates for PCCP execution so that adherence to the PCCP is properly executed and documented.
Requirements Organization: We often see engineering teams struggle to manage complicated trace matrixes in Excel. With Ultralight, customers are able to set up system architecture, quickly generate requirements with AI assistance, and properly organize and trace requirements to understand hierarchies and change dependencies.
Test Planning & Execution: Most AI/ML-enabled device teams struggle to keep up with the documentation and testing burden required in regulated algorithm development. At Ultralight, we help document and trace verification and validation testing, and also aim to automate test evidence generation and documentation.
While the pace of AI/ML advancement in medical devices has been impressive, the pace of software tools to serve these AI/ML product teams has been lacking. At Ultralight, we’ve purpose-built our software platform to make it dramatically easier for AI/ML-enabled medical device teams to design, develop, commercialize, and iterate on FDA-regulated algorithms.
If you’d like to learn more or see a demo, or learn more about PCCPs, reach out anytime.


